Does Dietitian Involvement During Pregnancy Improve Birth Outcomes? A Systematic Review
Abstract
Résumé
INTRODUCTION
METHODS
Search strategy
Study eligibility criteria
Data extraction
Study quality assessment
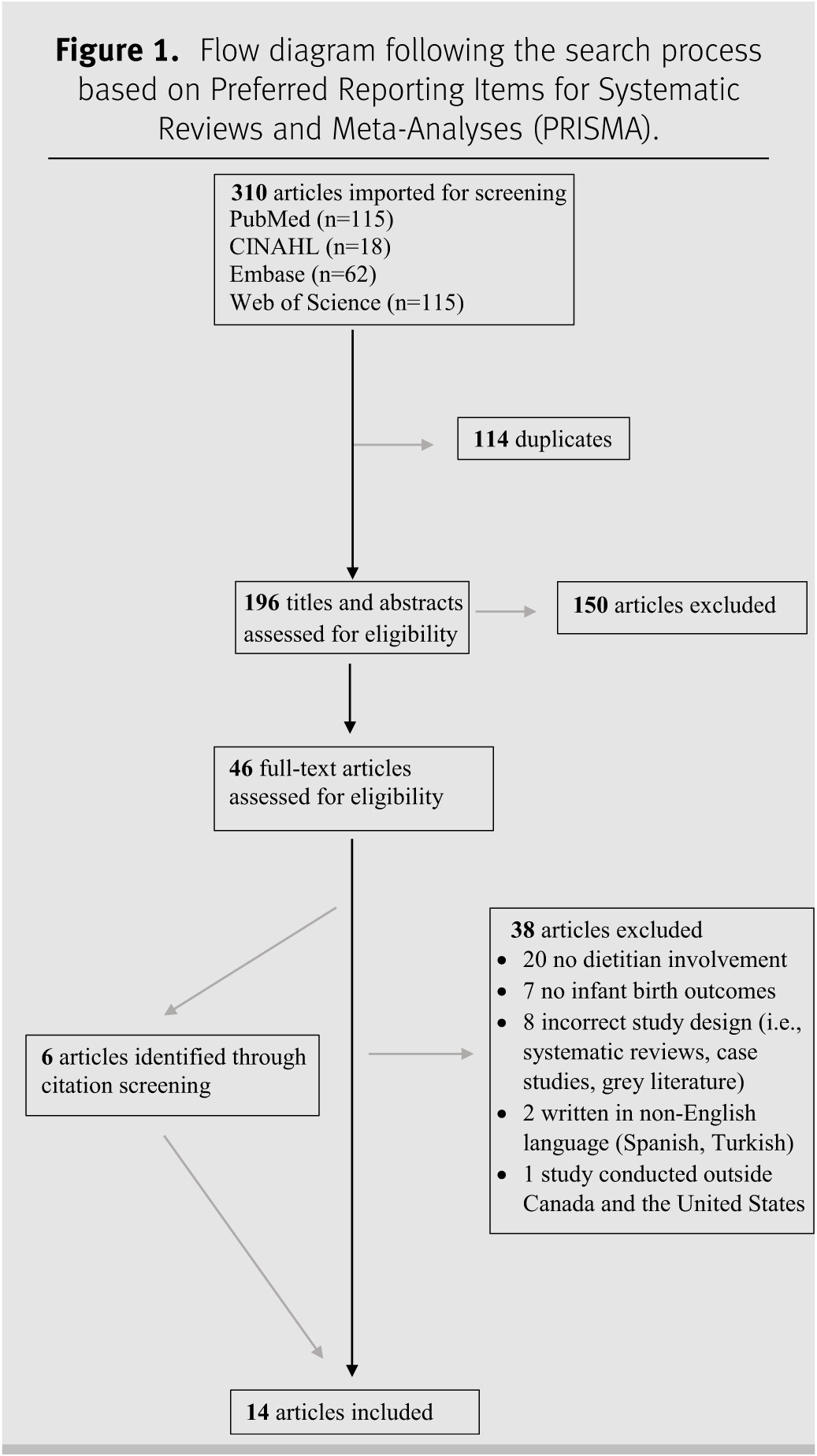
RESULTS
Description of studies
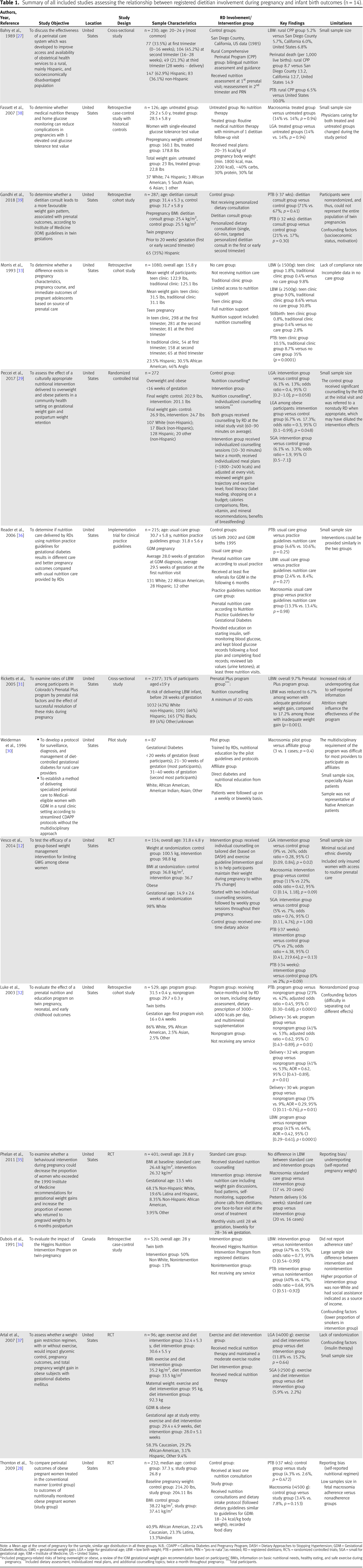
Low birth weight
Small for gestational age
Large for gestational age
Macrosomia
Preterm birth
Infant mortality
DISCUSSION
Main findings
Interpretation
Strengths and limitations
RELEVANCE TO PRACTICE
Footnote
REFERENCES
Supplementary Material
- Download
- 25.08 KB
Information & Authors
Information
Published In

History
Copyright
Key Words
Mots-clés
Authors
Metrics & Citations
Metrics
Other Metrics
Citations
Cite As
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
Cited by
View Options
View options
Get Access
Login options
Check if you access through your login credentials or your institution to get full access on this article.
Subscribe
Click on the button below to subscribe to Canadian Journal of Dietetic Practice and Research


