INTRODUCTION
Women experience many bodily changes throughout pregnancy to prepare for childbirth [
1], including increased caloric requirements, increased resting metabolic rate [
2], and weight gain [
3]. Weight gain can cause immense stress for pregnant women, especially among those with a history of eating disorders (EDs) [
4].
EDs are persistent disruptions in eating-related behaviour that lead to abnormal food consumption patterns or malabsorption of nutrients [
5]. Anorexia nervosa (AN), bulimia nervosa (BN), and binge-eating disorder (BED) are common EDs among young women in developed countries [
6]. AN is characterised by an intense fear of weight gain [
7], which can lead to extreme dieting, severe food restriction, and excessive exercise, or binge eating followed by purging [
5]. Complications of AN may include amenorrhea, loss of bone mineral density, and vital sign abnormalities [
8]. Diagnostic criteria for BN are similar to AN but include binge-eating and compensatory behaviours that occur at least once weekly for a minimum of 3 months [
5]. The age of onset for AN [
9] and BN [
10] is typically during adolescence and young adulthood [
11]. The main symptoms of BED include excessive caloric intake in a short period of time, accompanied by the individual’s sense of having lost control over their eating behaviour [
5].
A study by Easter et al. found that 7.5% of women in the United Kingdom had a diagnosed ED during pregnancy [
12]. Another study by Bulik et al. found that the prevalence of EDs during pregnancy was 0.2% for BN and 4.8% for BED [
13]. EDs may impact the body’s ability to absorb nutrients, which can have important implications for pregnancy and birth outcomes [
5,
14].
Systematic reviews have investigated the association between EDs and birth outcomes. das Neves et al. found that AN and BN are positively associated with low birth weight (LBW), and that BED is associated with higher birth weight and large for gestational age (LGA) [
1]. Another systematic review found that AN is associated with miscarriage and preterm birth (PTB), and that BN is associated with giving birth to a small for gestational age (SGA) infant [
15]. Reducing the risk of adverse birth outcomes is critical, as they increase the risk for infant morbidity and mortality, as well as the development of chronic health conditions in adulthood [
16]. For example, PTB is positively correlated with all-cause mortality in adulthood [
17], and both LBW and SGA increase the risk of type 2 diabetes [
18].
Currently, no meta-analyses exist that evaluate the relationship between EDs and adverse birth outcomes, thereby allowing for estimation of effect sizes for specific EDs and birth outcomes. This systematic review and meta-analysis examines the association between maternal AN, BN, and BED with the following birth outcomes: LBW, PTB, SGA, LGA, and miscarriage.
METHODS
This review was planned, conducted, and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses. The study’s protocol was registered on Open Science Framework (https://osf.io/myfbu).
Search strategy
Electronic literature searches were conducted in PubMed, CINAHL, Web of Science, and EMBASE databases. All authors were involved in developing the search strategy, with the help of a health sciences librarian. The search strategy encompassed all relevant literature using keywords (Supplementary Table 1
1) and subject heading terms adapted to each database. There were no restrictions on language, geographic region, date of publication, or study type. Reference lists of retrieved articles were also examined.
Study eligibility criteria
This review included primary, quantitative studies on women with a history of diagnosed AN, BN, and/or BED. Each study must have also examined the relationship between at least one of these EDs and at least one of the following birth outcomes:
•
PTB (<37 weeks gestation)
•
SGA (<10th percentile for birth weight)
•
LGA (>90th percentile for birth weight)
•
Miscarriage (fetal death before 20 weeks of pregnancy).
In addition, an ED diagnosis must have preceded the birth outcome. Qualitative studies, grey literature, articles not written in English, studies in which participants had disordered eating without an ED diagnosis, and studies in which the birth outcome preceded the ED diagnosis were excluded. Two authors (MM and NM) conducted independent title and abstract screening and full-text review with adjudication performed by the principal investigator (JAS) where required.
Study quality assessment
Two authors (MM and NM) independently assessed the methodological quality of nonrandomised studies using the Newcastle–Ottawa scale for cohort studies and case–control studies [
19]. Disagreements were resolved by consensus between the authors.
Data extraction
A data extraction sheet was developed using Google Sheets and Microsoft Excel (Microsoft Corporation, Redmond, WA) to retrieve data from each study. The extracted information included author names, year of publication, study design, sample size, geographic location, sociodemographic characteristics, pre-pregnancy body mass index (BMI), gestational weight gain, ED diagnosis, birth outcome(s) assessed, key findings, and study limitations. Two authors (MM and NM) independently coded each study to reduce coding bias.
Evidence synthesis
Two authors (MM and NM) conducted a vote count of all independent statistical tests. Specifically, vote counting was used to assess the relationship between EDs (AN, BN, and BED) and birth outcomes (miscarriage, PTB, LBW, SGA, and LGA) by comparing the number of studies with positive results to the number of studies with negative results. There were insufficient data to conduct a meta-analysis on BED and miscarriage, and BN and LBW. The vote counting focused on the direction of the findings of the main effects, regardless of the statistical significance level. In general, vote counting tends to be very conservative, by accepting the null hypothesis more easily than would be the case using meta-analytic methodology [
20]. Once the number of findings in each direction was counted, a sign test was used to assess the cumulative result, such that
Zvc = (
Np) – (½
N)/(½
√N), where
Zvc is the Z-score for the overall series of findings,
Np is the number of positive findings, and
N is the total number of findings (both positive and negative). Since the vote-count method does not provide information on effect size or sample size from each study, average odds ratios (OR) using unweighted and weighted effect sizes were computed using Review Manager (RevMan version 5.4, The Cochrane Collaboration), and a meta-analysis of the association between maternal EDs and adverse birth outcomes was conducted. Past and current EDs were analyzed as a single group to determine whether lifetime exposure to EDs was associated with adverse birth outcomes, irrespective of the time elapsed between ED recovery and pregnancy for those with past EDs. It was decided a priori that at least three papers were needed for a specific ED and birth outcome (e.g., AN and LBW) to include the pairing in the meta-analysis.
The Mantel–Haenszel random effects model was used to test the association between EDs and birth outcomes, and the OR was the effect measure. The random effects model assumes that both between-study variance and within-study error (i.e., sampling or estimation) are operating and produces larger confidence intervals (CIs), variances, and standard errors than fixed effects models. Random effects models give neither too much weight for studies with a large sample size nor too little weight for studies with a small sample size [
21]. Since the purpose of a meta-analysis is to combine studies and pool data to obtain a more precise estimate of effect, the
I2 statistic determined the percentage of variation across studies that is due to heterogeneity and not chance, with
I2 values of 25%, 50%, and 75% representing low, moderate, and high heterogeneity, respectively [
22]. While clinical heterogeneity always exists in meta-analyses (e.g., differences in patient characteristics, study settings, study design, study quality, exposures, and outcomes), the
I2 provides an assessment of statistical heterogeneity (e.g., inconsistency in findings between studies), where low
I2 values indicate less variability between studies.
RESULTS
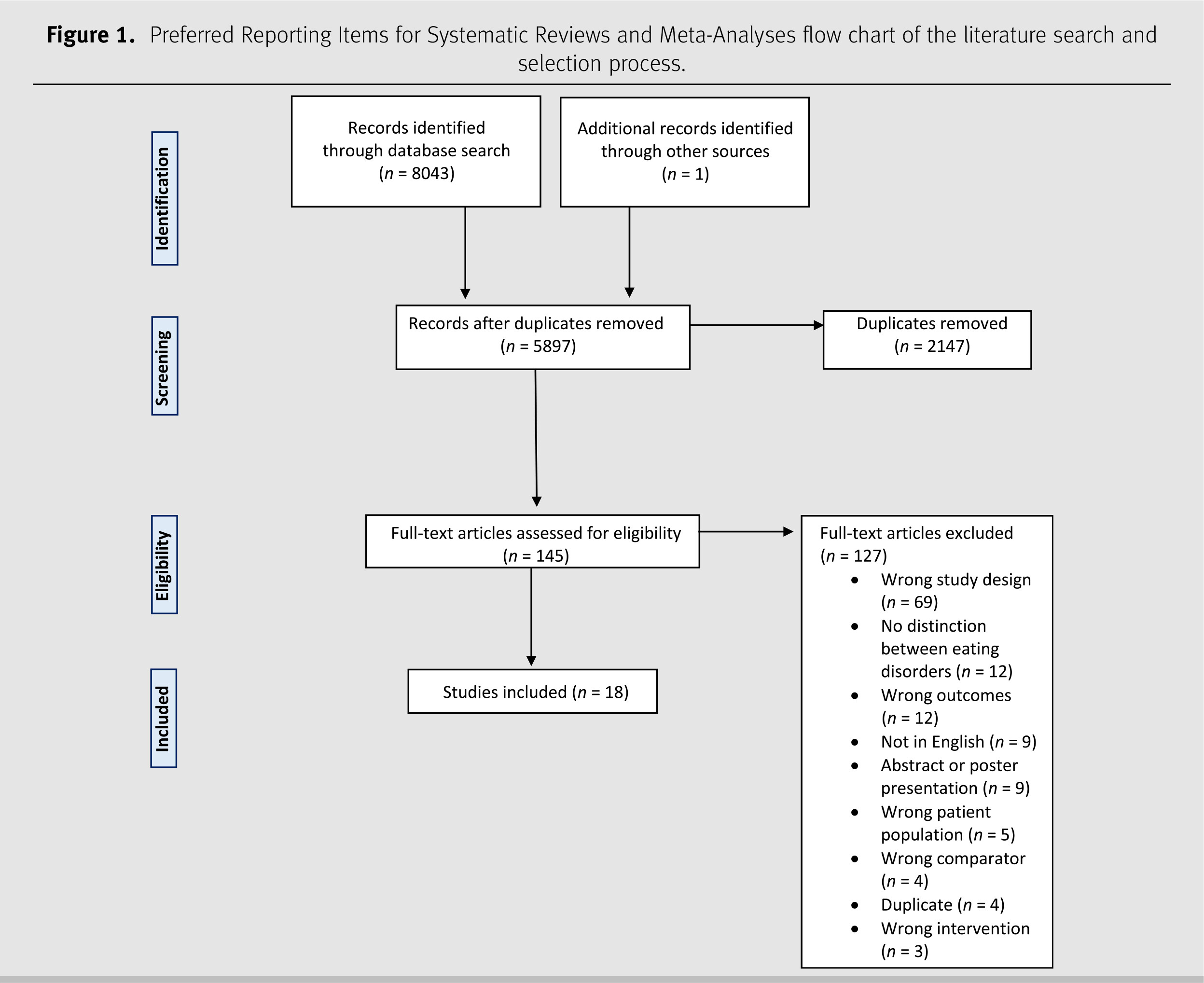
The database search resulted in 8,043 records. There were 2,147 duplicates removed and the remaining 5,896 articles were screened by title and abstract. Of those articles, 144 studies met the inclusion criteria for full-text review, and 18 were included (
Figure 1). Supplementary Table 2
1 summarises these articles. Most studies (95%,
n = 17) were cohort studies, and one was case control. The study sample size varied from 51 women [
23] to 2,134,495 women [
24]. Most studies were conducted in Europe (
n = 13), followed by Australia (
n = 2), the United States (
n = 2), and Canada (
n = 1). The publication years ranged from 1991 to 2020. AN was involved in 89% (
n = 16) of studies, 72% (
n = 13) involved BN, and 33% (
n = 6) BED.
Vote count: Maternal EDs and adverse birth outcomes
The results of the vote count show 5 of 5 positive findings between AN and miscarriage (p = 0.03), 7 of 12 for AN and PTB (p = 0.56), 4 of 5 for AN and LBW (p = 0.18), and 9 of 11 for AN and SGA (p = 0.04). With respect to studies on BN, 4 of 4 showed a positive relationship with miscarriage (p = 0.046), 7 of 11 with PTB (p = 0.37), 3 of 4 with LBW (p = 0.32), 7 of 9 with SGA (p = 0.10), and 3 of 5 with LGA (p = 0.66). Findings were positive in the only study on BED and miscarriage, 7 of 11 studies on BED and PTB (p = 0.45), and 4 of 5 studies on BED and LGA (p = 0.18).
Study quality assessment
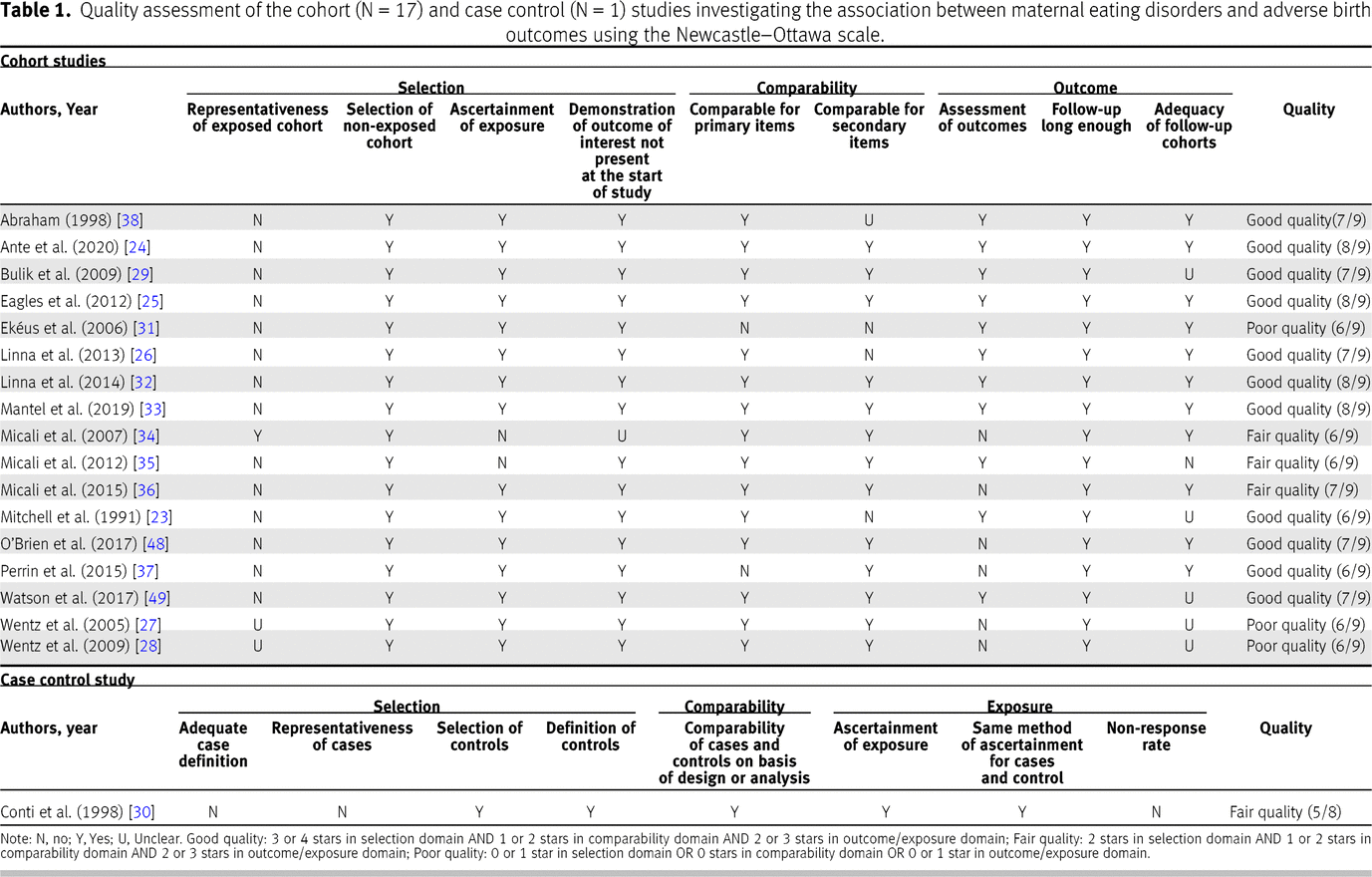
The results of the quality assessment (
Table 1) show that 11 of 18 studies were of good quality, 4 of 18 were of fair quality, and 3 were of poor quality.
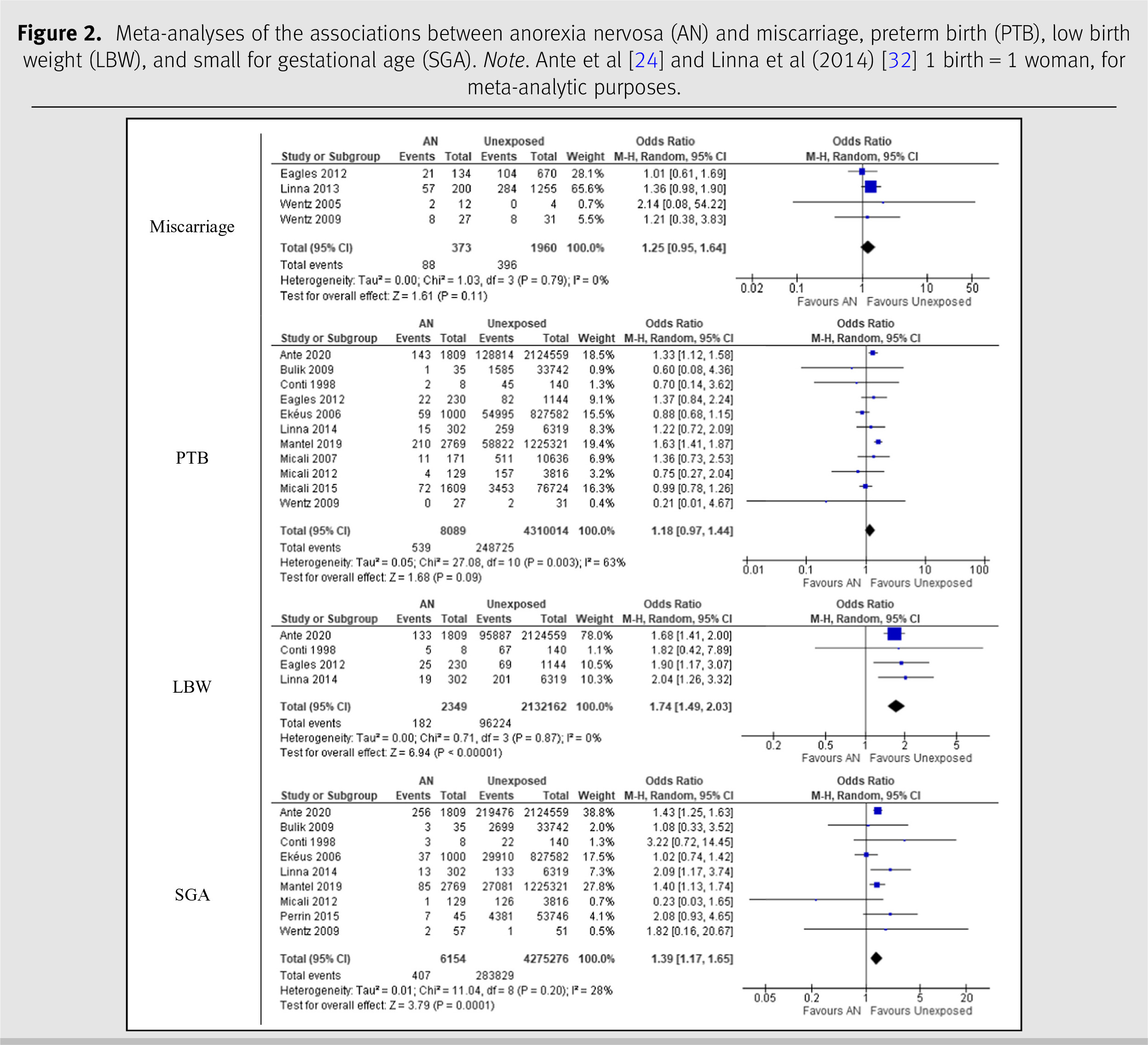
Anorexia nervosa
The meta-analyses on AN are shown in
Figure 2. The four studies on AN and miscarriage had a combined number of 2,333 participants [
25–
28]. The pooled OR comparing the prevalence of miscarriage in women with a history of AN was 1.25 (95% CI 0.95, 1.64) with an
I2 statistic of 0%. There were 11 studies on AN and PTB with a combined sample size of 4,318,103 women [
24,
25,
28–
36]. The pooled OR comparing the prevalence of PTB in AN was 1.18 (95% CI 0.97, 1.44) with an
I2 statistic of 63%. For LBW, the combined number of mothers with AN across the four studies was 2,134,511 [
24,
25,
30,
32]. The pooled OR comparing the prevalence of LBW in mothers with AN was 1.74 (95% CI 1.49–2.03) with an
I2 statistic of 0%. The nine studies on AN and SGA had a total of 4,281,430 participants [
24,
28–
33,
35,
37], a pooled OR of 1.39 (95% CI 1.17–1.65), and an
I2 statistic of 28%.
Bulimia nervosa
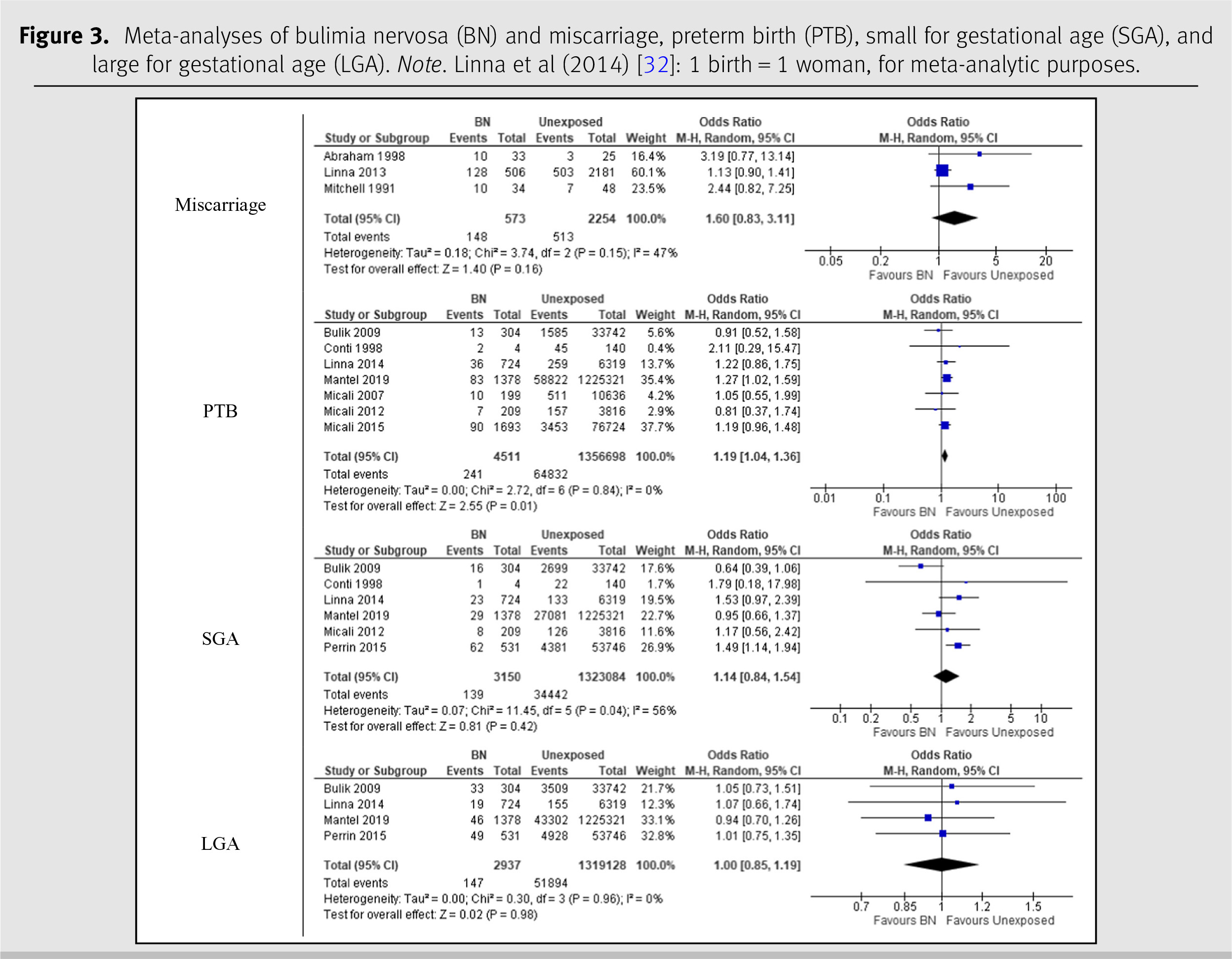
The meta-analysis on BN is displayed in
Figure 3. Three studies with a total of 2,827 participants were included in the analysis of BN and miscarriage [
23,
26,
38]. The pooled OR for this association was 1.60 (95% CI 0.83, 3.11) with an
I2 statistic of 47%. The seven studies on BN and PTB had a combined number of 1,361,209 participants [
29,
30,
32–
36]. The pooled OR for these studies was 1.19 (95% CI 1.04, 1.36), and the
I2 statistic was 0%. The meta-analysis included six studies on BN and SGA with a total of 1,326,234 participants [
29,
30,
32,
33,
35,
37]. The pooled OR was 1.14 (95% CI 0.84–1.54), with an
I2 statistic of 56%. The analysis on BN and LGA was conducted using four studies with a combined number of 1,322,065 participants [
26,
29,
33,
37]. The analysis yielded a pooled OR of 1.00 (95% CI 0.85, 1.19) and an
I2 statistic of 0%.
Binge-eating disorder
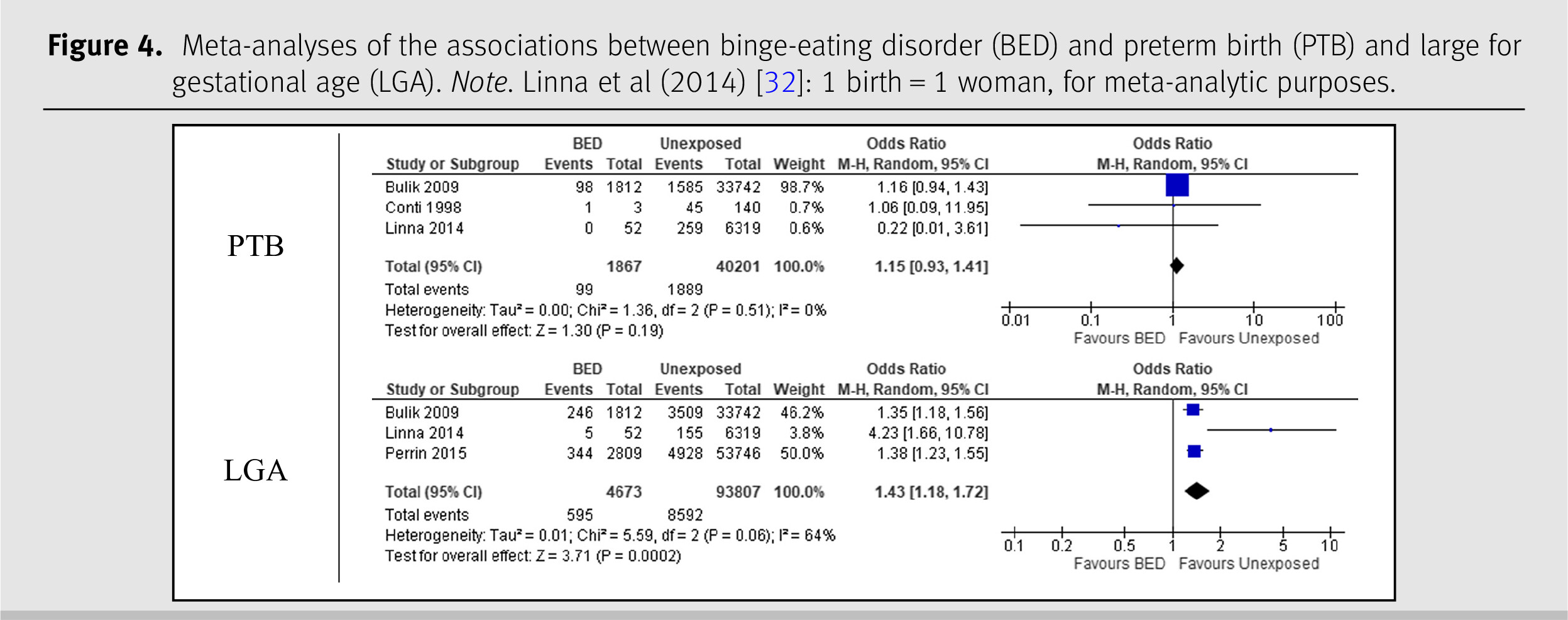
The meta-analysis on BED and PTB included three studies with a combined sample size of 42,068 women [
29,
30,
32]. The pooled OR comparing the prevalence of PTB among women with BED was 1.15 (95% CI 0.93–1.41) and the
I2 statistic was 0%. The meta-analysis on BED and LGA included three studies and 98,480 participants [
29,
32,
37]. The analysis yielded an OR of 1.43 (95% CI 1.18–1.72) and an
I2 statistic of 64%. Both results are shown in
Figure 4.
CONCLUSION
This systematic review and meta-analysis found that AN was positively correlated with LBW and SGA, BN with PTB, and BED with LGA. Factors that may influence birth outcomes within ED populations, such as disease status during pregnancy (active or remittent) and gestational weight gain, should be investigated. Future research on maternal BED and birth outcomes is also needed.
Financial support: None.
Conflict of interest: The authors declare that they have no competing interests.